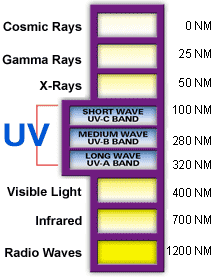
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, that is, in the range 10 nm to 400 nm, corresponding to photon energies from 3 eV to 124 eV. It is so-named because the spectrum consists of electromagnetic waves with frequencies higher than those that humans identify as the colour violet. These frequencies are invisible to humans, but visible to a number of insects and birds.
UV light is found in sunlight (where it constitutes about 10% of the energy in vacuum) and is emitted by electric arcs and specialized lights such as black lights. It can cause chemical reactions, and causes many substances to glow or fluoresce. Most ultraviolet is classified as non-ionizing radiation.
 The discovery of UV radiation was associated with the observation that silver salts darkened when exposed to sunlight. In 1801, the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum darkened silver chloride-soaked paper more quickly than violet light itself. He called them "oxidizing rays" to emphasize chemical reactivity and to distinguish them from "heat rays," discovered the previous year at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favour of ultraviolet and infrared radiation, respectively
The discovery of UV radiation was associated with the observation that silver salts darkened when exposed to sunlight. In 1801, the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum darkened silver chloride-soaked paper more quickly than violet light itself. He called them "oxidizing rays" to emphasize chemical reactivity and to distinguish them from "heat rays," discovered the previous year at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favour of ultraviolet and infrared radiation, respectively
The higher energies of the ultraviolet spectrum from wavelengths about 10 nm to 120 nm ('extreme' ultraviolet) are ionizing, but this type of ultraviolet in sunlight is blocked by normal dioxygen in air, and does not reach the ground. However, the entire spectrum of ultraviolet radiation has some of the biological features of ionizing radiation, in doing far more damage to many molecules in biological systems than is accounted for by simple heating effects (an example is sunburn). These properties derive from the ultraviolet photon's power to alter chemical bonds in molecules, even without having enough energy to ionize atoms.
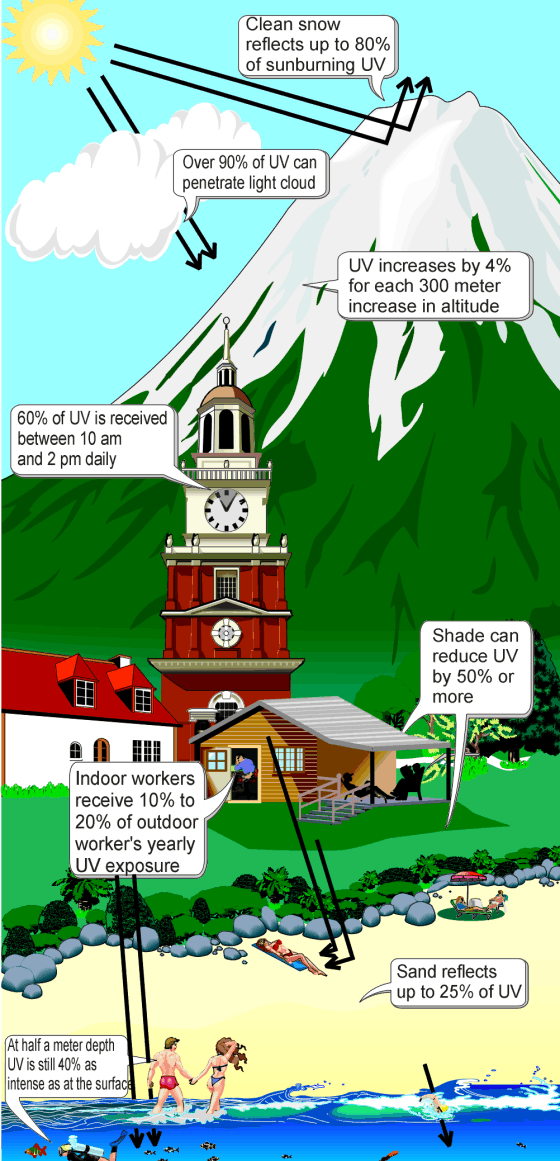
Although ultraviolet radiation is invisible to the human eye, most people are aware of the effects of UV on the skin, called suntan and sunburn. In addition to short wave UV blocked by oxygen, a great deal (97%) of mid-range ultraviolet (almost all UV above 280 nm and most above 315 nm) is blocked by the ozone layer, and like ionizing short wave UV, would cause much damage to living organisms if it penetrated the atmosphere.
After atmospheric filtering, only about 3% of the total energy of sunlight at the zenith is ultraviolet, and this fraction decreases at other sun angles. Much of it is near-ultraviolet that does not cause sunburn. An even smaller fraction of ultraviolet that reaches the ground is responsible for sunburn and also the formation of vitamin D (peak production occurring between 295 and 297 nm) in all organisms that make this vitamin (including humans). The UV spectrum thus has many effects, both beneficial and damaging, to human health.
The most common form of UV radiation is sunlight, which produces three main types of UV rays:
- UVA
- UVB
- UVC
However, the longer wave UVA and UVB can cause molecules to vibrate and rotate resulting in heating up. The shorter wave UVC (used in UV Sterilization) light will ionize many atoms and molecules as compared to the even shorter wave Gamma Rays which will ionize most atoms. Ultra violet sterilization is an effective tool for disease prevention in aquariums, ponds and for general water quality control such as control of Green Ponds or Cloudy Aquariums. As well the use of UVC Sterilization is useful in home, office, hospital air purification ( even UVC/Redox Blood therapy).
There is a lot of new evidence as to the benefits of UV sterilization for ALL fish, and many myths have been dispelled such as “UV Sterilizers destroying beneficial nitrifying bacteria”.
One of the most powerful methods of sterilizing any medical instrument that may penetrate the skin is through the use of ultraviolet light. Ultraviolet light used for sterilizing medical equipment is composed of light frequencies in the C band of the ultraviolet spectrum, otherwise known as UV-C. Sterilizing equipment is simple, but all surfaces must be exposed to the UV-C light for a specific amount of time. UV disinfection is a photochemical process. The microorganisms that pollute the indoor environment are DNA or RNA based. The cell membranes and DNA break down when exposed to high intensity UV at 253.7nm. This bond breakage translates into cellular membrane damage in which case the cells dye or DNA/RNA damage which renders the germs harmless because they can no longer reproduce and cause disease.
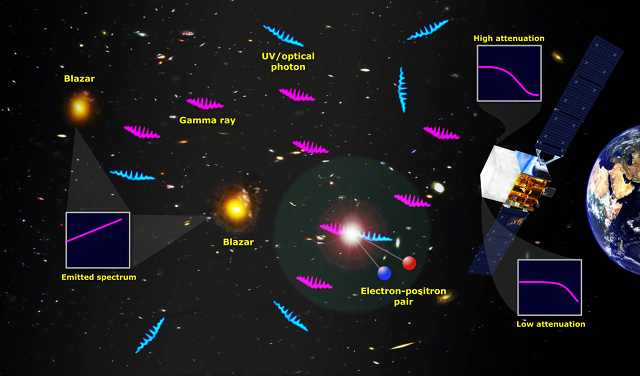 Cosmically speaking, Very hot objects emit some amount of UV radiation. The hotter the object, the more UV is emitted. Observing and recording the UV from astronomical objects such as planets in our solar system, stars, nebulae and galaxies enables us to gain extra information such as the temperature and chemical composition of these objects. The only problem is that our Earth’s ozone layer absorbs much of the UV and so these observations need to be made outside the Earth’s atmosphere. On board the Hubble Space Telescope, the Faint Object Spectrograph (FOS) and the Goddard High Resolution Spectrograph (GHRS) are used to collect and analyse UV light from interesting targets.
Cosmically speaking, Very hot objects emit some amount of UV radiation. The hotter the object, the more UV is emitted. Observing and recording the UV from astronomical objects such as planets in our solar system, stars, nebulae and galaxies enables us to gain extra information such as the temperature and chemical composition of these objects. The only problem is that our Earth’s ozone layer absorbs much of the UV and so these observations need to be made outside the Earth’s atmosphere. On board the Hubble Space Telescope, the Faint Object Spectrograph (FOS) and the Goddard High Resolution Spectrograph (GHRS) are used to collect and analyse UV light from interesting targets.Astrochemistry is the study of the abundance and reactions of chemical elements and molecules in the universe, and their interaction with radiation. One particularly important experimental tool in astrochemistry is spectroscopy, the use of telescopes to measure the absorption and emission of UV light from molecules and atoms in various environments.
Our solar system Hydrogen is easily detected in the ultraviolet (UV) and visible ranges from its absorption and emission of light. This type of detection allows us to seek out sources of water or even other chemicals which could indicate the ingredients of life.
UV light maybe taken for granted and become synonymous with tanning and solar activity, but thanks to science it has become an important tool in sterilization and Astrochemistry. Perhaps with further study we can study the effects of DNA mutation and chemical interaction on the nano scale. The future of Ultra violet light can be so much more then disco lights and tanning booths, hopefully it wont be taken for granted any more...





No comments:
Post a Comment